VET Veterinary syringe pump for animal use rechargeable battery injection pump

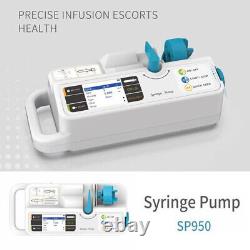










VET Veterinary syringe pump for animal use rechargeable battery injection pump. SP950 is a syringe pump for medical use, and it adopts microcomputer system and microelectronic technology, features in high-intelligence and high-safety. LCD can display each parameter in real-time, with multi-function alarm, and alarm with visual, auditory and character prompt.
It takes the advantages of easy operation, steady work, high accuracy and checking the syringe specification automatically. And now, it has been widely applied in each vein injection in hospitals for clinical nursing, such as ICU, CCU, neonatology, obstetrics and gynecology, pediatrics, internal medicine, surgery, operating room, emergency room. 1 Infusion rate and total volume can be set by user. 2 Alarms for infusion pipe occlusion, syringe fall-off, communication abnormal, low battery.
3 Bright, alarm volume and range of pressure alarm are adjustable. 4 Quick feed function, convenient for supplying medicine quickly and instantaneously. 5 Enter KVO mode after infusion completed.
6 The system will give prompt signal when there is no operation at power-on state. 7 Built-in rechargeable battery provides such functions as auto-switch power supply and auto-charge. 8 Display parameters with large screen, and give prompt information with visual, auditory and character signal, which is convenient for medical persons to operate. 9 The device can equipped with disposable syringe (CNS), and accuracy can be ensured by adjusting parameters. 1 Specification requirement for syringe: 10ml/20ml/30ml/50ml (CNS). 3 Rate of quick feed. 4 Accumulated volume display: 0ml-999.9ml.6 Pressure range for occlusion alarm. 7 KVO rate: 0.1ml/h5ml/h, adjustable. Equipment, internally powered type BF. 10 Maximal power consumption: 20VA30VA, the device can continuously work for at least two hours with the injection rate of 5ml/h or 500ml/h after fully charged.
11 Power supply: AC100240V 50/60Hz. 12 Battery: rechargeable lithium battery, 7.4V, 3500mAh. 13 Working environment: Temperature: +540?Atmospheric pressure: 860hPa1060h; Relative humidity: 20%90%; no strong vibration and no corrosive gas. 14 Storage environment: Temperature: -20? Relative humidity: =90%, no condensation; No corrosive gas and well-ventilated. The sale of this item may be subject to regulation by the U.
Food and Drug Administration and state and local regulatory agencies. The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG) with the code 197923, and certified by FDA of United States and CE, TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved. We are looking forward to establishing a successful business relationship with you. Only english user guide, if you need any other language, please contact me.This item is in the category "Business & Industrial\Healthcare, Lab & Dental\Medical, Lab & Dental Supplies\Syringes". The seller is "contechealthcare" and is located in this country: US. This item can be shipped to United States.
- Brand: CONTEC
- Syringe Tip: Slip Tip
- Intended Use/Discipline: Biological Laboratory, Dental Laboratory, Emergency Medicine, Internal Medicine, Medical Laboratory, Physical Laboratory, Physical Medicine & Rehabilitation, Veterinary Medicine
- Model: SP950VET
- Country/Region of Manufacture: China
- Power supply:: Rechargeable Battery
- Instrument classification:: Class II
- Weight:: 1.7kg
- Dimension:: 310mm*140mm*135mm(L×W×H)
- Type:: Medical Equipment
- warranty: 1 year warranty
- After-sale Service:: Repair and Replacement