Mallinckokrodt OptiScan LE Dual Head Injector System

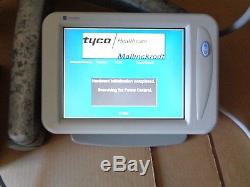


Sale is for a Mallinckokrodt OptiScan LE Injector System.. S hipping is Free via Ground Insured.. 1 - Mallinckokrodt OptiScan LE Injector System. System is missing the Data/Power Cable that connects from the power unit to the power control Cable is normally 75' to 150' depending on the distance from the control room to the magnetic room.. Also a filter P/N 801223 may be required in some locations. Also stand base has stains to the aluminum.. 2 of the legs on the Power Control Unit are missing(See Last Picture). This Sale is available to authorized Buyers Only... Needs to be re-certified by a authorized professional before placing into service. The sale of this item may be subject to regulation by the U.
Food and Drug Administration and state and local regulatory agencies. All equipment should be inspected by a qualified licensed Company or Professional and be certified prior to use.
Fill out section A or B (A Medical Facilities / B Device Resellers). In the prescribed use of the medical device items identified below.
I also certify that prior to sale. Of use of such devices I will take necessary steps to assure that such devices are not adulterated. Or misbranded within the meaning of those terms in the Federal Food, Drug and Cosmetic Act. In the manufacture and/or refurbishing of the medical device items identified below. Certify that prior to sale ofuse of such devices I will take necessary steps to assure that such.Devices are not adulterated or misbranded within the meaning of those terms in the Federal. Food, Drug and Cosmetic Act. The item "Mallinckokrodt OptiScan LE Dual Head Injector System" is in sale since Sunday, February 19, 2017. This item is in the category "Business & Industrial\Healthcare, Lab & Life Science\Medical Equipment\Other Medical Equipment".
The seller is "jedcoy2010" and is located in Southwest. This item can be shipped to United States.