CONTEC CA10S VET End-tidal Capnograph ETCO2 Veterinary use CO2 Measurement

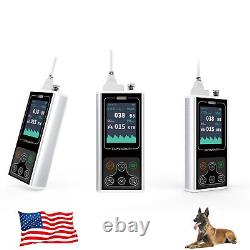




The device adopts sidestream method to measure the animal's parameter related to EtCO2 and AwRR. It can detect pulmonary ventilation function and reflect the circulation and pulmonary blood flow and indirectly reflect the alveolar ventilation.
It is applicable for use in pre-hospital emergency, emergency department, ICU, operating room, respiratory department and other occasions. Features 1End-tidal CO2 measurement function; 2Respiration rate measurement function; 3Optional three units; 4With audible and visual alarm and silencing functions, the alarm limit can be set; 5Over-limit alarm function for EtCO2, Respiration rate; 6Low battery alarm function; 7With the prompt function of "Checking Filterline"; 8User can calibrate zero by themselves. 9With atmospheric pressure and temperature compensation functions 10Rechargeable lithium battery, up to 8-hour user time; Performance End-tidal CO2 measurement: 1Measurement range: 0 150mmHg 2Resolution: 1mmHg 3Accuracy: 0~40 mmHg:±2 mmHg 41~70 mmHg:±5% of readings 71~100 mmHg:±8% of readings 101~150 mmHg:±10% of readings Respiration rate measurement: 1Measurement range: 2rpm~120rpm 2Resolution: 1rpm 3Accuracy: ±1rpm Safety characteristic: 1Device type: internally powered equipment 2Degree of protection against electric shock: type BF applied part 3Degree of protection against ingress of liquid: IPX1 Other parameters: 1Preheat time: 2min; 2Response time.
Food and Drug Administration and state and local regulatory agencies. The Fingertip Pulse Oximeter is registered on the Australian Register of Therapeutic Goods (ARTG)with the code 197923, and certified by FDA of United States and CE, TUV of Europe. The Fingertip Pulse Oximeter that is FDA 510K Approved. These charges are buyers' responsibility. We are look for partners in the world now! Do you want to become our agent in you conutry?