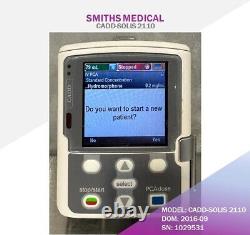
Cadd-solis 2110 Ambulatory Infusion Pump
The sale of this item may be subject to regulation by the U. Food and Drug Administration and state and local regulatory agencies. Reprocessing will be required before patient use. It is up to the purchaser to meet all requirements and regulation certification processes that deemed necessary per FDA or any other regulatory agencies that may be applicable. As with any piece of medical equipment, this unit needs to be inspected and calibrated before being put into use.